AT-Closed Vial® and Container Closure Integrity during Cryogenic Storage
More
We use cookies to offer you the best experience on our site. You can find out more about the cookies we use or disable them in the Cookie settings
Each injection to your patient relies on the compliant aseptic filling process without contamination and the safe administration procedures on the hospital site. The tubing systems and devices in direct contact with the drug product, known as product path, are essential for the success of the filling operations.
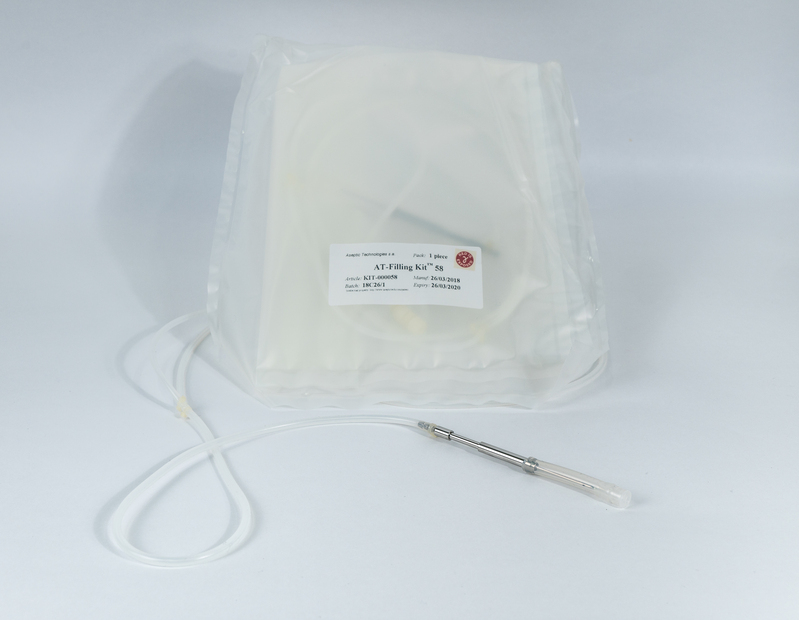
The AT-Assembly is a customizable, single-use, disposable fluid path system for aseptic filling operations with the AT-Closed Vial® technology, and aseptic fluid transfer operations.
Combined to our Filling Lines, the AT-Assembly provides :
The standdard AT-Assembly consists of a specific filling needle and a system of tubing, for ergonomic transfer from the bulk product container to the filling unit, trough the peristaltic dosing system.
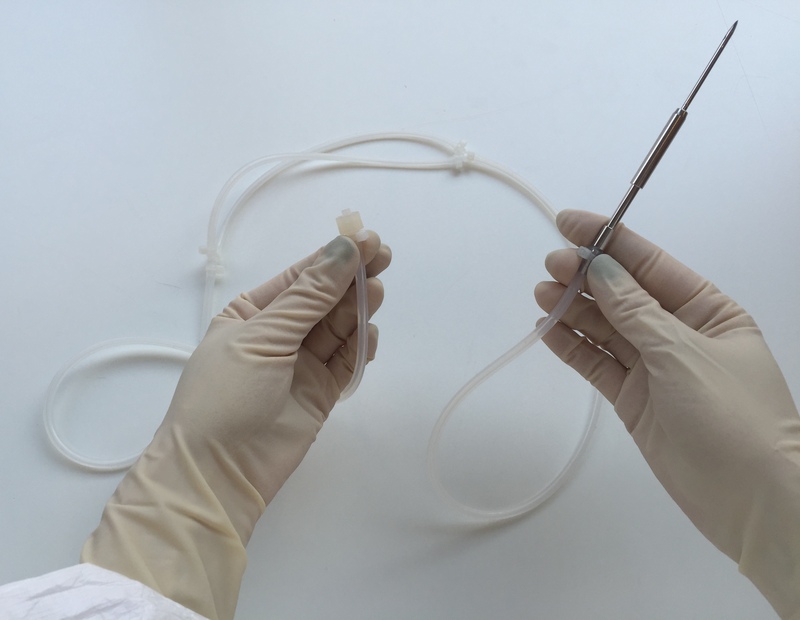
With over 20 years of experience in single-use assemblies, we leverage our double innovative expertise in aseptic processes and production of said assemblies to offer you custom cleanroom solutions that meet your needs.
We take care of the design and prototyping of the assembly, the production in qualified cleanrooms, the sterilization process validation (gamma-irradiation), the release testing, quality support and compliance documentation.



To secure the clinical outcomes, the therapy developers need to take into consideration the product administration procedures. User-friendly SOPs and devices enable error-proof administration, increasing patient safety.
To faster bring your injectable therapy in AT-Closed Vial® to market, we designed the AT-Adapt, a dedicated stand-alone disposable device for vial access without use of a needle. The device is intended for use by healthcare professionals in a wide variety of healthcare environments, including hospitals and pharmacies.
The AT-Adapt is available for the 2 ml, 6ml, 10 ml, 20 ml, 50 ml formats of the AT-Closed Vial®.
AT-Closed Vial® and Container Closure Integrity during Cryogenic Storage
More